SAVVE® U.S. Pivotal Trial: One Year Definitive Data
%
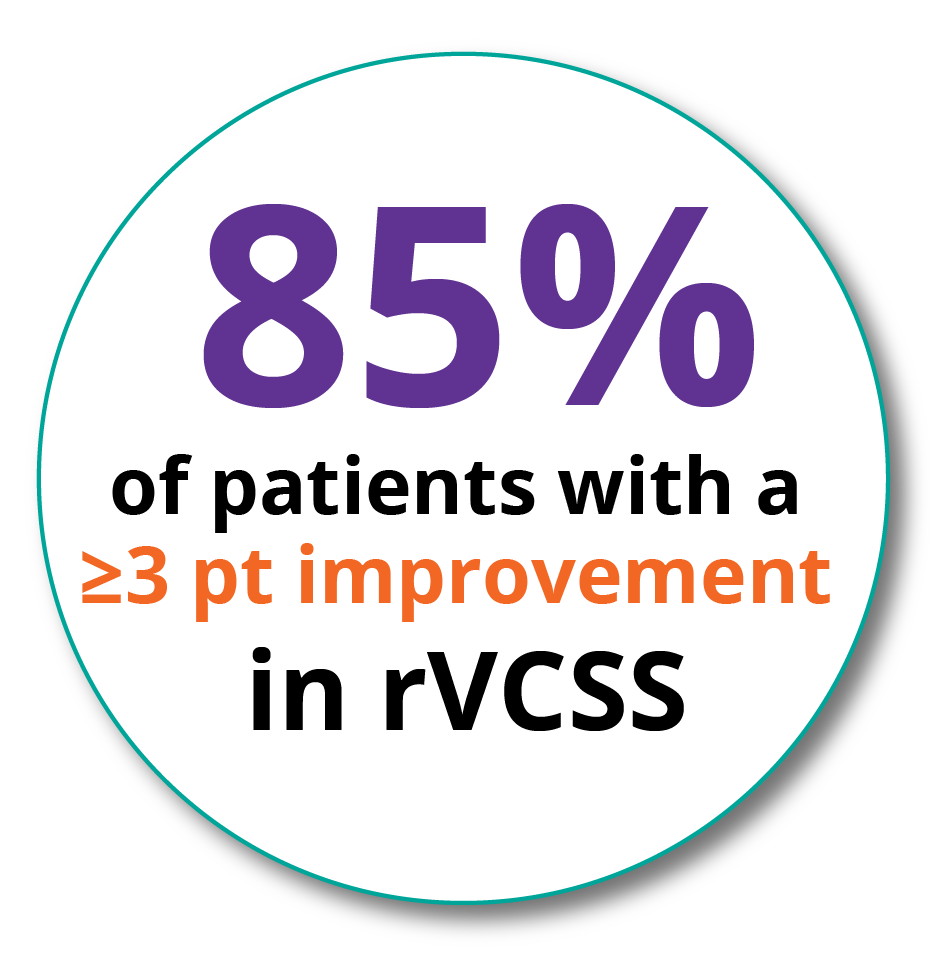
Clinical Meaningful Benefit Responder Rate*
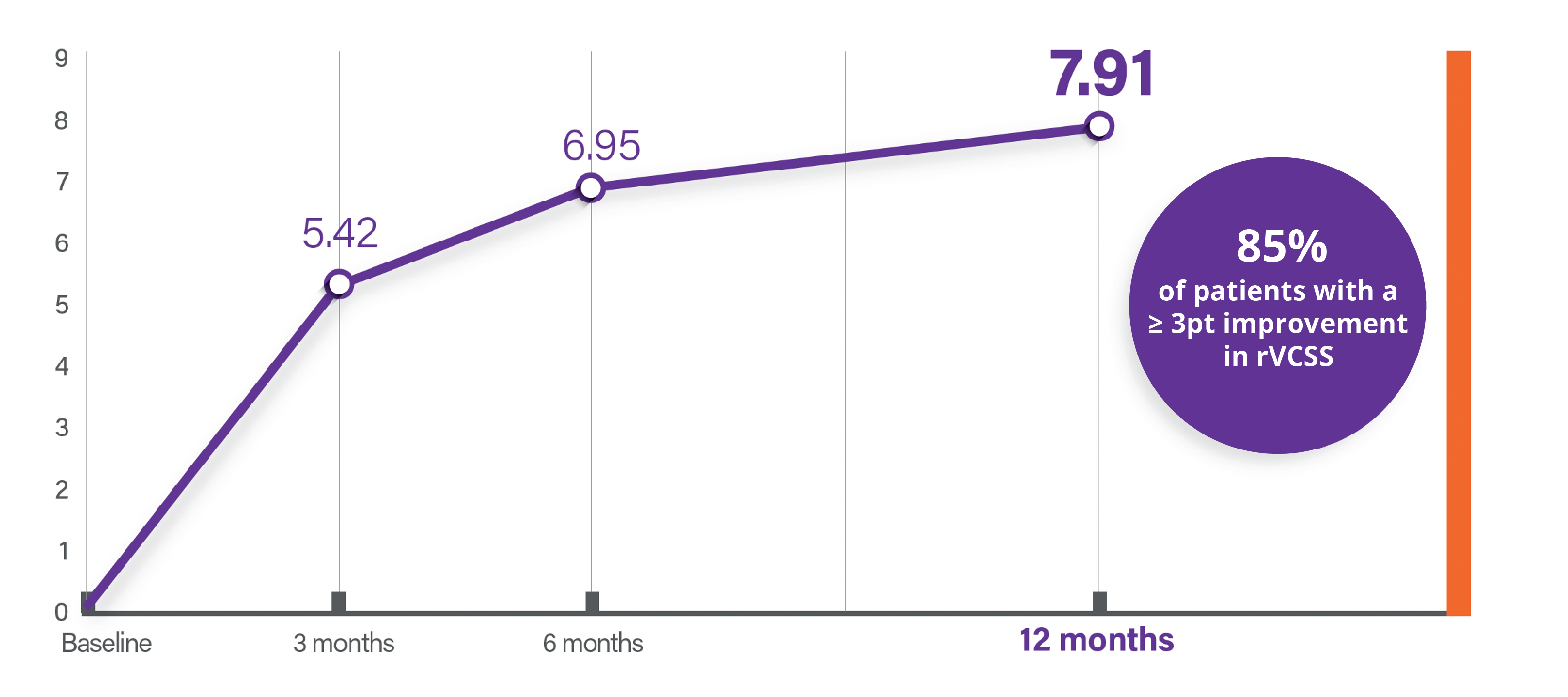
Point Average rVCSS Improvement**
%
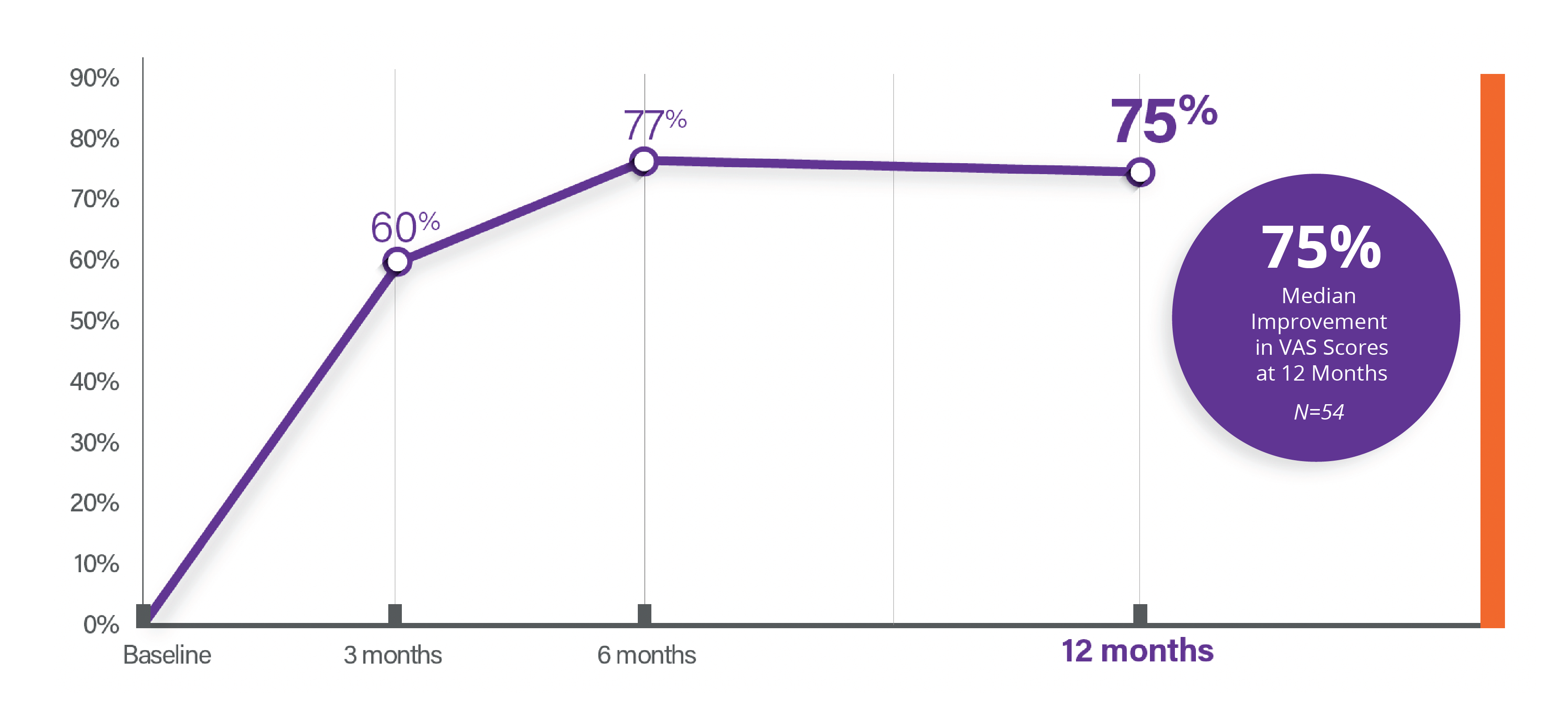
Median Reduction in Pain (VAS)
%
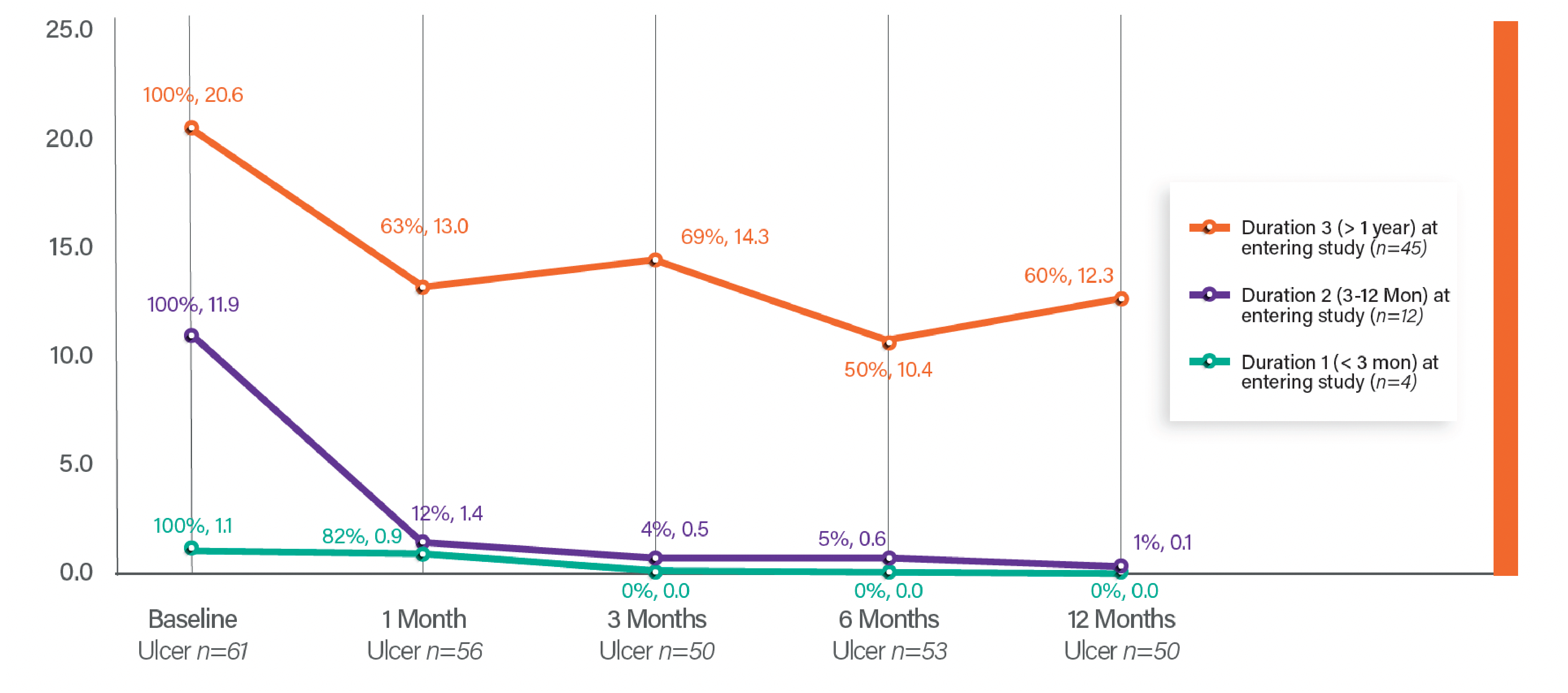
Median Ulcer Area Reduction
%
Target Vein Patency Rate
*Clinically meaningful benefit of a three (3) or more point improvement in revised Venous Clinical Severity Score (rVCSS)
**For the clinically meaningful benefit responder cohort
VenoValve® Implantation Procedure
Improvement in Patient Reported Quality-of-Life Indicators
%
VEINES Symptom Score*
%
VEINES Qol Score*
*Mean (± SD): The VEINES scoring system consists of two composite scores – one for quality of life and one for symptom severity
rVCSS: Clinically Meaningful Benefit
Percentage of Patients with Clinical Meaningful Benefit at Follow Up
- 3 Months 71%
- 6 Months 74%
- 12 Months 85%
rVCSS Point Improvement
Point Improvement in Average rVCSS Clinical Meaningful Benefit
Click the image to enlarge
rVCSS is a clinically validated scoring system used to track the regression or progression of venous diseases
Are you a physician with interest in the VenoValve?
Pain (VAS) – One Year
Percentage Ulcer Reduction
Patency
Target Vein Patency Rate
Device Patency
30 DAYS
12 MONTHS
12 MONTHS
%
%
%
Target Vein Patency Rate
30 DAYS
%
12 MONTHS
%
Device Patency
12 MONTHS
%
Procedural Outcomes
PROCEDURAL
SUCCESS RATE
INTRA-OPERATIVE
DEVICE PATENCY
%
%
Procedural Outcomes
PROCEDURAL
SUCCESS RATE
%
INTRA-OPERATIVE
DEVICE PATENCY
%
Major Adverse Events (MAEs) at 1 Year
-
One (1) Death (unrelated to the VenoValve) ⎮ Zero (0) Pulmonary Embolisms ⎮ Twelve (12) Target Vein Thromboses ⎮ Ten (10) Surgical Pocket Hematomas ⎮ Four (4) Other Bleeds ⎮ Seven (7) Deep Wound Infections
-
Percent (94%) of the subjects who experienced an MAE (not including the unrelated death) also experienced a clinical meaningful benefit
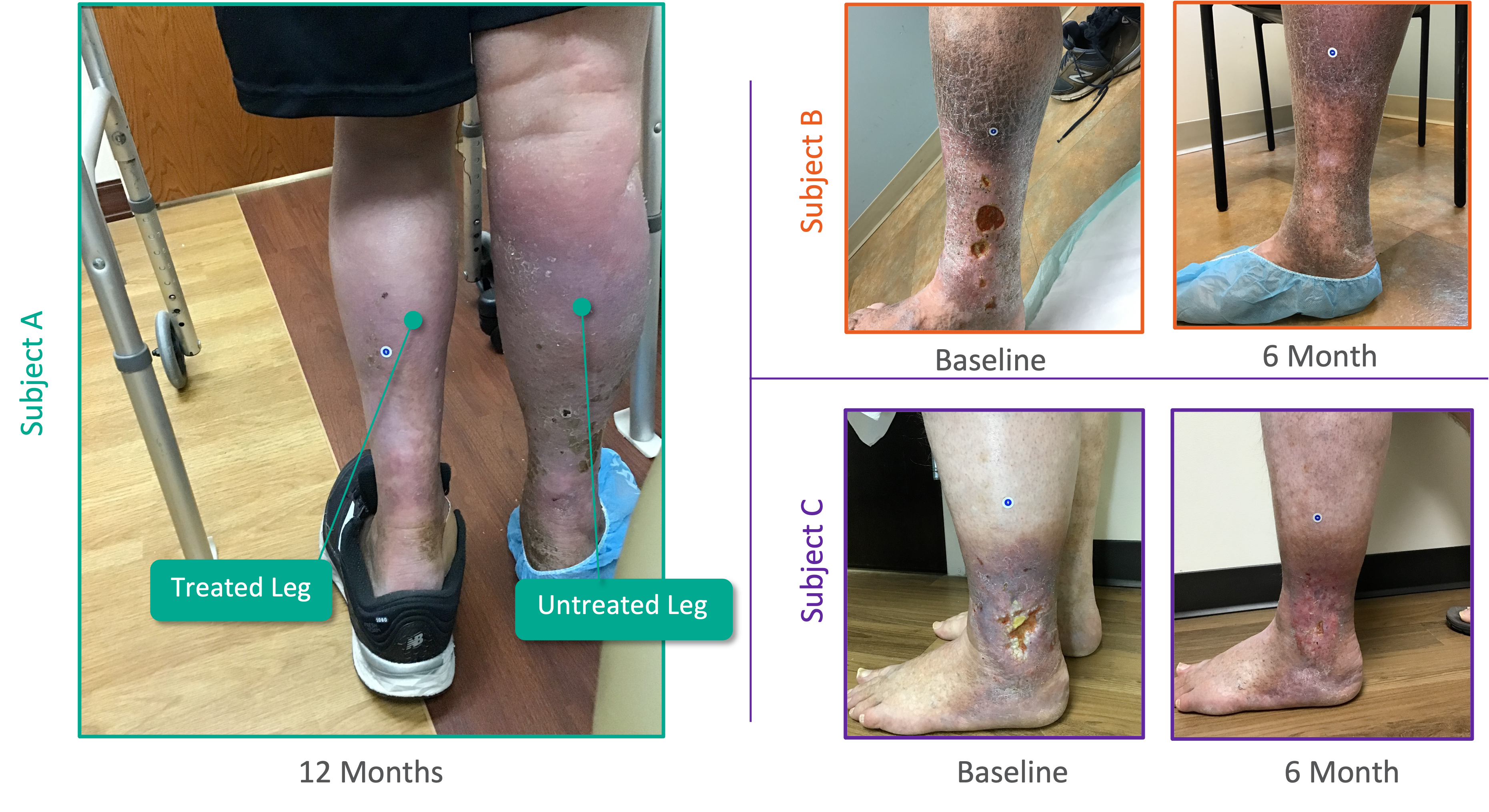
Patient Characteristics
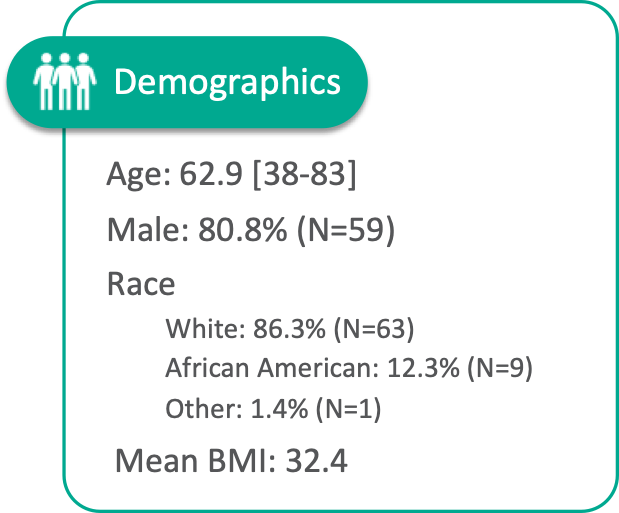
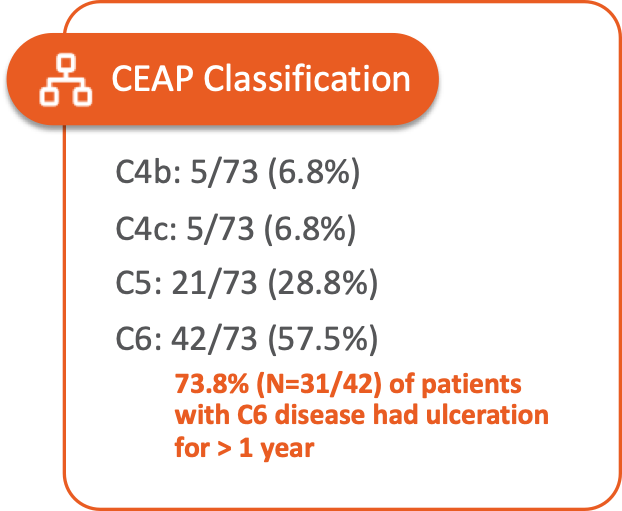
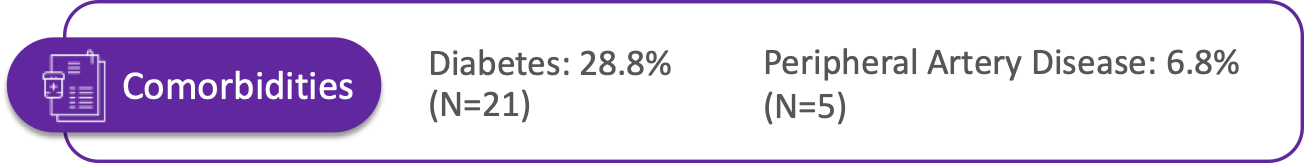
The SAVVE® US Pivotal Trail enrolled 75 patients across 21 clinical sites. The device has not been approved for marketing or sale in the United States or elsewhere.
Active Sites
Albany Medical Center
391 Myrtle Ave,
Albany, NY 12208
PI Name: Dr. Adriana Laser
Primary Contact: Brenda Romeo
Cedars-Sinai Medical Center
La Cienega Blvd.
Beverly Hills, CA 90211
PI Name: Dr. NavYash Gupta
Primary Contact: Denice Dubuclet
Houston Methodist Hospital
6550 Fannin St.
Houston, TX 77030
PI Name: Dr. Eric Peden
Primary Contact: Kikelomo Akindoju
Indiana University
1801 North Senate Blvd., D3500
Indianapolis, IN 46202
PI Name: Dr. Raghu Motaganahalli
Primary Contact: Janet Klein
Jobst Vascular Institute /
Promedica Toledo Hospital
2109 Hughes Drive, CJT STE. 400
Toledo, OH 43606
PI Name: Dr. Gregory Kasper
Primary Contact: Christy Terrasi
Kaleidahealth/University at Buffalo
100 High St.
Buffalo, NY 14203
PI Name: Dr. Linda Harris
Primary Contact: Robin Stein
Lankenau Medical Center
100 E Lancaster Ave.
Wynnewood, PA 19096
PI Name: Dr. Vincent DiGiovanni
Primary Contact: Shefali Bansal
Miami Vascular Specialists
8950 N. Kendall Drive
Miami, FL 33176
PI Name: Dr. Michele Taubman
Primary Contact: Keivy Garcia
NYU Langone
530 1st Ave, HCC6F
New York, NY 10016
PI Name: Dr. Mikel Sadek
Primary Contact: Gina Bernardez
Pima Heart and Vascular
2404 E River Road, Suite 100
Tucson, AZ 85718
PI Name: Dr. Scott S Berman
Primary Contact: Aleksander Herber (520) 576.4133
Saint Louis University
1008 S Spring Ave.
Saint Louis, MO 63110
PI Name: Dr. Matthew Smeds
Primary Contact: (314) 977.4730
St. Peter's Vascular Associates
2 New Hampshire Ave.
Troy, NY 12180
PI Name: Dr. Kathleen Ozsvath
Primary Contact: Debbie Goodman
Sentara
600 Gresham Drive
Norfolk, VA 23507
PI Name: Dr. David Dexter
Primary Contact: Sarah Have
The Mount Sinai Hospital
1190 Fifth Avenue
New York, NY 10029
PI Name: Dr. Windsor Ting
Primary Contact: Sarah McCracken
TriHealth
375 Dixmyth Avenue
Cincinnati, OH 45220
PI Name: Dr. Patrick Muck
Primary Contact: Melody Vespie
University of Alabama at Birmingham
Vascular Surgery and Endovascular Therapy
BDB 652, 1808 7th Ave. South
Birmingham, AL 35294
PI Name: Dr. Marc Passman
Primary Contact: Rebecca St. John
University of Chicago
5841 S. Maryland Ave., MC 5028
Chicago, IL 60637
PI Name: Dr. Chelsea Dorsey
Primary Contact: MacKenton Johnson
University Hospitals Cleveland
Medical Center
11100 Euclid Ave.
Cleveland, OH 44106
PI Name: Dr. Karem Harth
Primary Contact: Emily Mullenax
University of Louisville
550 South Jackson St.
Louisville, KY 40202
PI Name: Dr. Amit Dwivedi
Primary Contact: Leslie Haysley (502) 852.2801
UPMC
5200 Centre Avenue
Pittsburgh, PA 15232
PI Name: Dr. Rabih Chaer
Primary Contact: Julianna Sheline
University of Utah
30 N. 1900 East, Room 3C344 SOM
Salt Lake City, UT 84132
PI Name: Dr. Claire L. Griffin
Primary Contact: Julie Hales
Vanderbilt University Medical Center
D-5237 Med Center North,
1161 21st Ave. South
Nashville, TN 37232
PI Name: Dr. Mark Iafrati
Primary Contact: Celia Nunez
Yale New Haven Hospital
330 Cedar St, Boardman 204
New Haven, CT 06510
PI Name: Dr. Cassius Iyad Ochoa Chaar
Primary Contact: Edgar Benitez
Participating Clinical Sites
Participating Clinical Sites

Active Sites
Albany Medical Center
391 Myrtle Ave,
Albany, NY 12208
PI Name: Dr. Adriana Laser
Primary Contact: Brenda Romeo
Cedars-Sinai Medical Center
La Cienega Blvd.
Beverly Hills, CA 90211
PI Name: Dr. NavYash Gupta
Primary Contact: Denice Dubuclet
Houston Methodist Hospital
6550 Fannin St.
Houston, TX 77030
PI Name: Dr. Eric Peden
Primary Contact: Kikelomo Akindoju
Indiana University
1801 North Senate Blvd., D3500
Indianapolis, IN 46202
PI Name: Dr. Raghu Motaganahalli
Primary Contact: Janet Klein
Jobst Vascular Institute /
Promedica Toledo Hospital
2109 Hughes Drive, CJT STE. 400
Toledo, OH 43606
PI Name: Dr. Gregory Kasper
Primary Contact: Christy Terrasi
Kaleidahealth/University at Buffalo
100 High St.
Buffalo, NY 14203
PI Name: Dr. Linda Harris
Primary Contact: Robin Stein
Lankenau Medical Center
100 E Lancaster Ave.
Wynnewood, PA 19096
PI Name: Dr. Vincent DiGiovanni
Primary Contact: Shefali Bansal
Miami Vascular Specialists
8950 N. Kendall Drive
Miami, FL 33176
PI Name: Dr. Michele Taubman
Primary Contact: Keivy Garcia
NYU Langone
530 1st Ave, HCC6F
New York, NY 10016
PI Name: Dr. Mikel Sadek
Primary Contact: Gina Bernardez
Pima Heart and Vascular
2404 E River Road, Suite 100
Tucson, AZ 85718
PI Name: Dr. Scott S Berman
Primary Contact: Aleksander Herber (520) 576.4133
Saint Louis University
1008 S Spring Ave.
Saint Louis, MO 63110
PI Name: Dr. Matthew Smeds
Primary Contact: (314) 977.4730
St. Peter's Vascular Associates
2 New Hampshire Ave.
Troy, NY 12180
PI Name: Dr. Kathleen Ozsvath
Primary Contact: Debbie Goodman
Sentara
600 Gresham Drive
Norfolk, VA 23507
PI Name: Dr. David Dexter
Primary Contact: Sarah Have
The Mount Sinai Hospital
1190 Fifth Avenue
New York, NY 10029
PI Name: Dr. Windsor Ting
Primary Contact: Sarah McCracken
TriHealth
375 Dixmyth Avenue
Cincinnati, OH 45220
PI Name: Dr. Patrick Muck
Primary Contact: Melody Vespie
University of Alabama at Birmingham
Vascular Surgery and Endovascular Therapy
BDB 652, 1808 7th Ave. South
Birmingham, AL 35294
PI Name: Dr. Marc Passman
Primary Contact: Rebecca St. John
University of Chicago
5841 S. Maryland Ave., MC 5028
Chicago, IL 60637
PI Name: Dr. Chelsea Dorsey
Primary Contact: MacKenton Johnson
University Hospitals Cleveland
Medical Center
11100 Euclid Ave.
Cleveland, OH 44106
PI Name: Dr. Karem Harth
Primary Contact: Emily Mullenax
University of Louisville
550 South Jackson St.
Louisville, KY 40202
PI Name: Dr. Amit Dwivedi
Primary Contact: Leslie Haysley (502) 852.2801
UPMC
5200 Centre Avenue
Pittsburgh, PA 15232
PI Name: Dr. Rabih Chaer
Primary Contact: Julianna Sheline
University of Utah
30 N. 1900 East, Room 3C344 SOM
Salt Lake City, UT 84132
PI Name: Dr. Claire L. Griffin
Primary Contact: Julie Hales
Vanderbilt University Medical Center
D-5237 Med Center North,
1161 21st Ave. South
Nashville, TN 37232
PI Name: Dr. Mark Iafrati
Primary Contact: Celia Nunez
Yale New Haven Hospital
330 Cedar St, Boardman 204
New Haven, CT 06510
PI Name: Dr. Cassius Iyad Ochoa Chaar
Primary Contact: Edgar Benitez